How to Build HIPAA Compliant Mobile App? Explore Top Healthcare Laws & Regulations Applying to Your Healthcare App in the USA

2 years ago
This is the most simplified blog ever written on developing a HIPAA-compliant mobile and web app.
And here is what you’ll gain from this blog.
- A complete understanding of the US healthcare laws and regulations
- Find the right healthcare laws that apply to your healthcare app idea.
- Our HIPAA-compliant app development execution (which we have been performing for 8+ years.)
So, let’s come straight to the point.
A quick overview of US Healthcare laws and regulations
This is the first and crucial step in order to build a HIPAA compliant mobile and web app.
Because understanding healthcare laws and regulations is essential – if you want to make your healthcare app compliant with all the data privacy and security laws.
So, let’s get started.
1) Who regulates the healthcare industry?
The USA is one of the largest and most flourishing healthcare industries.
But jumping into it is not as easy as it sounds.
Multiple healthcare laws and federal approval make it one of the toughest tasks to launch any healthcare app in the US market.
Healthcare laws in the USA apply to Health Information Technology (HIT), mobile health, personalized prescriptions, wearable technology, and telehealth.
When it comes to regulating the US healthcare industry, three government bodies play a major role.
These government bodies are,
- Food and Drug Administration (FDA)
- Federal Trade Commission (FTC)
- Office of Civil Rights (OCR)
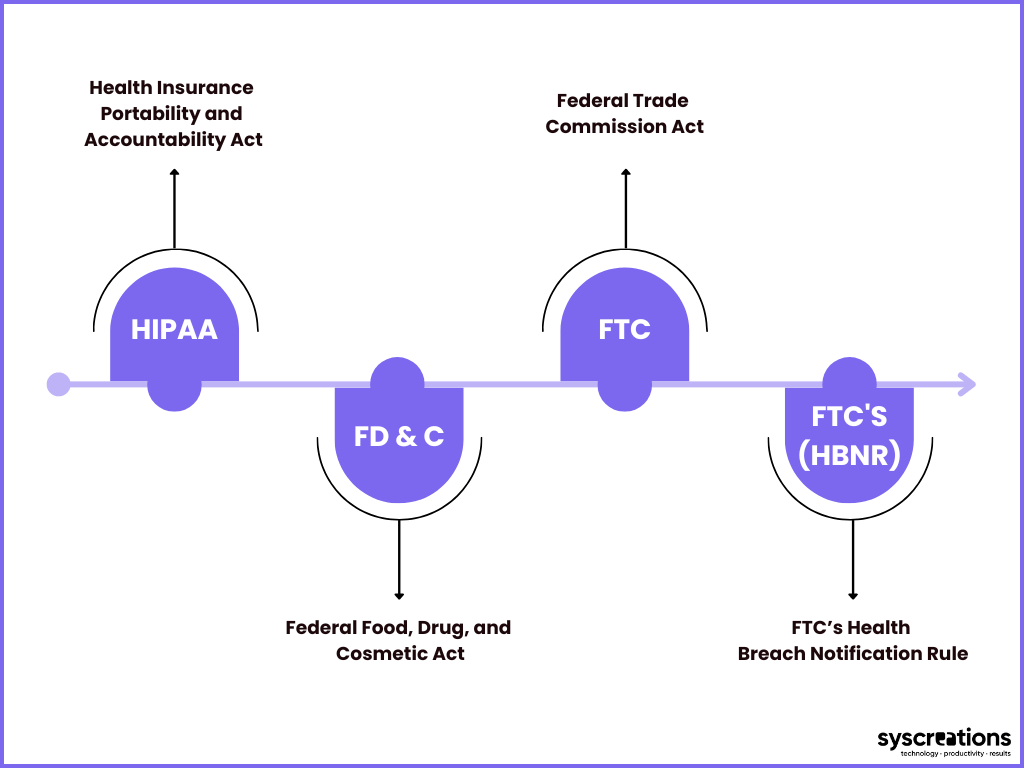
2) What are the top healthcare laws in the USA?
You should adhere to relevant healthcare law(s) if your healthcare mobile app collects, creates, and shares consumer information.
The following are the top 4 healthcare laws applicable in the USA.
How to build a HIPAA-compliant mobile and web app in the USA?
Developing a HIPAA-compliant healthcare app is not rocket science.
What you simply need to do is – identify the healthcare laws and regulations that apply to your healthcare mobile and web app idea.
Once you are aware of it, just keep those laws in mind during the app development process – rather say, the coding lifecycle.
We’ve listed out a few questions that will help you identify the healthcare laws that apply to your healthcare mobile app.
Question 1: Do you create, receive, and save the personal information of patients?
If Yes,
Go to Question 2 to know if HIPAA applies.
If No,
HIPAA does not apply. (But the FD&C Act might apply. Go to Question 5 to verify.)
Question 2: Are you a healthcare provider?
If Yes,
HIPAA applies. (Go to Question 5 to see if the FD&C Act also applies.)
If No,
HIPAA might apply. (Go to Question 3 to validate.)
Question 3: Is it mandatory for users to have a prescription to access your app?
If Yes,
HIPAA applies. (The FD&C Act might apply as well. Go to Question 5 to verify.)
If No,
HIPAA might apply. (Go to Question 4 to validate.)
Question 4: Are you developing this app on behalf of the hospital, doctor’s office, or health insurer?
If Yes,
You are called HIPAA Business Associate.
Thus, you are subject to HIPAA Security Rule and HIPAA Privacy and Breach Notification Rules.
But the FTC’s Health Breach Notification Rule does not apply.
(The FD&C Act might apply as well. Go to Question 5 to verify.)
If No,
HIPAA does not apply.
FTC’s Health Breach Notification Rule might apply. Go to Question 9 to validate.
(But the FD&C Act might apply. Go to Question 5 to verify.)
Question 5: Are the diagnosis of a disease, its treatment, and prevention of disease major uses of your app?
If Yes,
Your app is a medical device.
Thus, the FD&C Act applies.
(Go to Question 6 to see if the FDA gives you an exception.)
If No,
The FD&C Act does not apply.
(But the FTC Act might apply. Go to Question 8 to validate.)
Question 6: Does your app fall under the “minimal risk” category?
If Yes,
FD&C Act does not apply.
(FDA gives you an exception.)
Your app is falling under the ‘minimal risk’ category,
- If it is helping users to manage their healthcare condition by themselves without offering treatment suggestions.
- If it is offering very straightforward tools to users to keep an eye on their health information and track it.
- If it is automating day-to-day tasks for healthcare providers.
- If it is facilitating users or healthcare providers to interact with the EHR system.
If No,
FD&C act applies.
(Go to Question 7 to see if the FDA still gives you an exception.)
Question 7: Is your app a mobile medical app?
If Yes,
FDA gives you an exception.
(But the FTC Act might apply. Go to Question 8 to validate.)
Your mobile app falls under the mobile medical app category,
- If it acts as an accessory to a regulated medical device.
- If it transfers the mobile device into a regulated medical device such as a glucose meter.
- If it analyzes data from another medical device.
If No,
Please contact the FDA at mobilemedicalapps@fda.hhs.gov to validate whether FDA Act applies or not.
(FTC Act might apply as well. Go to Question 8 to validate.)
Question 8: Are you a nonprofit organization?
If Yes,
The FTC Act does not apply.
(But FTC’s Health Breach Notification Rule might apply. Go to Question 9 to validate.)
If No,
The FTC Act Applies.
(FTC’s Health Breach Notification Rule might apply as well. Go to Question 9 to validate.)
Question 9: Are you providing health records directly to customers?
If Yes,
The FTC’s Health Breach Notification Rule does not apply.
(You don’t have to now answer Question 10.)
If No,
FTC’s Health Breach Notification Rule might apply. Go to Question 10 to validate.
What are the US Healthcare laws and regulations?
1. HIPAA Act
HIPAA talks about healthcare compliance rules in 4 major categories.
- HIPAA Privacy Rule
The HIPAA Privacy Rule ensures the privacy of the personal information of patients and their family members.
It makes it compulsory for healthcare providers to put strict measures in effect to collect, share and save the ePHI safely.
HIPAA Privacy Rule also sets limits on the uses and disclosures of data without the permission of users.
This rule enables users to examine and obtain a copy of their health records.
- HIPAA Security Rule
The HIPAA Security Rule promotes the technical and physical measures to assure the confidentiality, integrity, and availability of electronic PHI.
- HIPAA Breach Notification Rule
Under the HIPAA Breach Notification Rule, healthcare providers must identify the data breach and provide notification or make affected users, the Secretary of HHS, and even the media (in some cases) familiar with the breach incident.
The HHS has developed an online portal to submit the breach incident details.
- HIPAA Omnibus Rule
The HIPAA Omnibus rule was finalized by HHS in 2013.
According to the rule, business associates are now directly liable for any non-compliance.
It also controls the use of ePHI for marketing purposes.

2. Federal Food, Drug, and Cosmetic Act (FD&C Act)
The FD&C Act is enforced by the FDA.
It governs the safety and effectiveness of medical devices and mobile apps.
The aim to enforce this rule is to ensure that all medical devices which include mobile apps are safe for public use.
FDA has developed a category named the mobile medical app.
So, if your app is a mobile medical app, your app does not fall under this jurisdiction.
3. Federal Trade Commission Act (FTC Act)
The FTC Act is enforced by the Federal Trade Commission.
It restricts false claims over the app’s safety, privacy, and performance.
In other words, it defines regulatory protocols to cope with unfair claims in businesses and issues related to privacy and general data security challenges.
4. FTC’s Health Breach Notification Rule
FTC’s Health Breach Notification Rule makes it mandatory for healthcare providers to provide notifications if they encounter a data breach.
Under the FTC’s Health Breach Notification Rule, healthcare providers who experience a data breach must notify the affected individuals, media, and FTA.
This rule does not apply to healthcare providers covered by HIPAA.
This Rule requires the personal health record vendors and their related entities to notify consumers about a breach involving unsecured information.
Additionally, if a service provider connected to any entity encounters a data breach, he/she must notify the entity resulting in them notifying the consumers.
Read our latest blog covering the actions taken by the FTC on encountering data breaches here.
How do we build HIPAA-compliant mobile and web apps?
Based in Ontario, we are a healthcare-focused IT company.
In other words, we only entertain healthcare IT projects for startups, hospitals, clinics, organizations, and individuals.
What distinct us from the rest is that – we accommodate dedicated compliance experts for each project we perform.
And our lead compliance specialist has designed a result-oriented plan to build HIPAA-compliant mobile apps in the USA, Canada, and beyond.
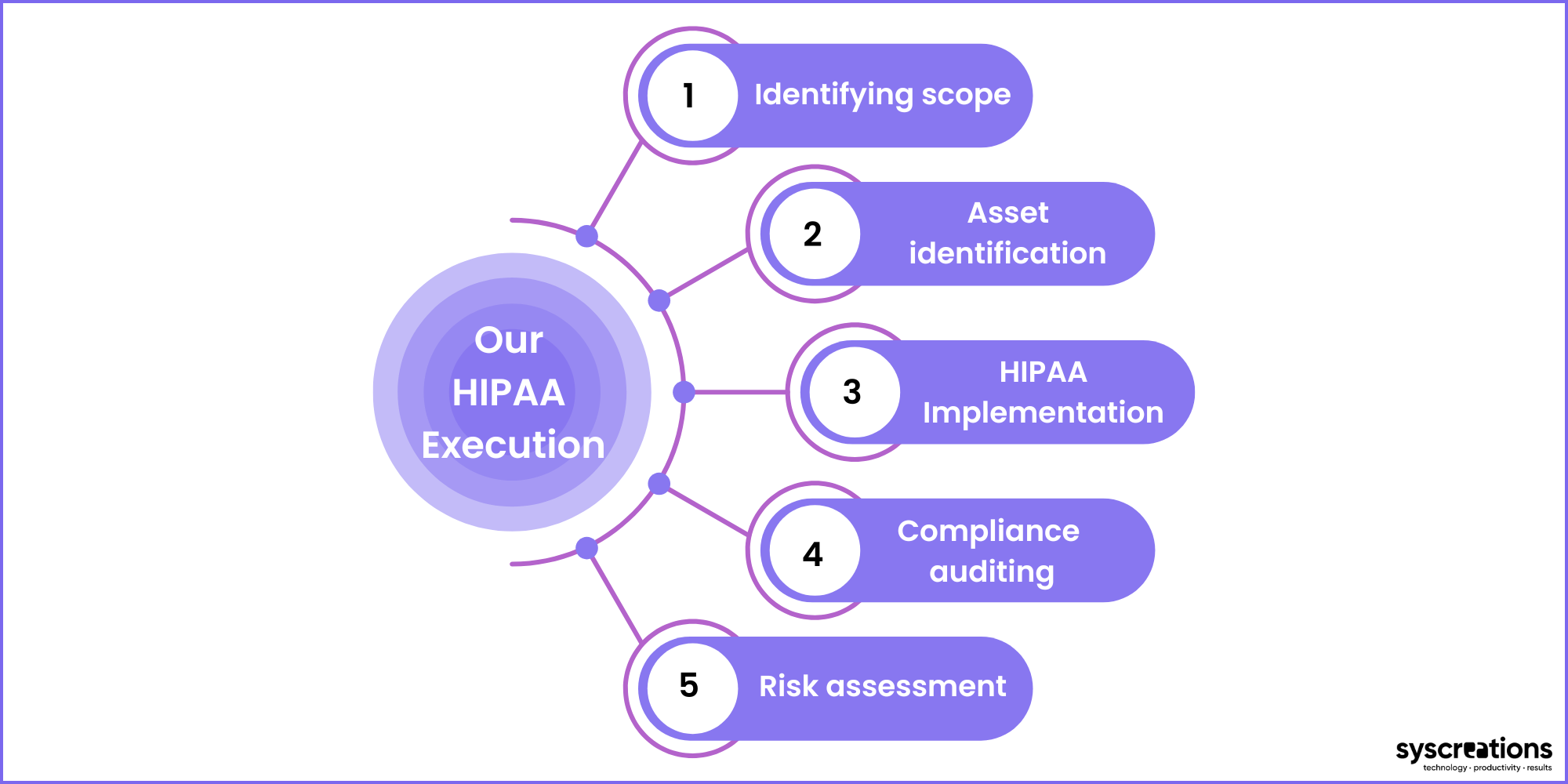
Following is the entire process.
1. Identifying the scope
We first determine the need for HIPAA compliance.
It may include – technical, administrative, and physical safeguards.
2. Asset location, asset identification, and risk analysis
It is important to have a clear understanding of the scale of healthcare app infrastructure.
Without knowing it – it is almost impossible to identify how much control your app requires to protect your app against cyber attacks.
But this step helps us to identify each security loophole your app possesses.
3. Implementation
Here, our HIPAA compliance experts work with the developers' team.
They keep their bulls-eyes open during the entire development lifecycle along with ensuring that all your technical and administrative compliance requirements are met.
4. Compliance auditing
This is a method to ensure,
- A successful implementation
- Achieved safety standards
Once this audit is done, you can claim that your mobile or web app is HIPAA compliant.
5. Risk assessment
Healthcare apps are always surrounded by cyber criminals – there is no doubt about it.
Thus, it is important to perform a risk assessment regularly to identify and fulfill the security gaps in your app.
This approach adds an extra layer of security to your app and enables you to avoid data breaches and hefty fines from the federal government.
What is the importance of developing a HIPAA-compliant mobile and web app?
There are two major reasons why healthcare regulations are so important for healthcare app development.
- Prosperous industry
As far as the data is concerned, healthcare is the most prosperous industry.
In addition to the personal information of patients and their family members, a healthcare device, mobile app, or software collects sensitive financial information of the patients.
The large number of people who access healthcare services and share their crucial personal and financial information also makes it important to impose rules to ensure data security and data privacy.
- A soft target
Though the healthcare industry is one of the late adopters of modern technologies, they adopted them rapidly.
Because of the rapid adoption, it failed to address the gray area of the technologies which resulted in a lack of cybersecurity and even IT system knowledge among users interacting with those modern technologies.
Such users are the greatest threat to healthcare data and the greatest gift to intruders!
7 Steps for Applying HIPAA to your mobile and web app
1. Get access control
Your app should impose restrictions on who can alter and see patients' confidential information.
No one should see more than the required patient information according to HIPAA Privacy Rules.
2. A secure entity or person authentication
You need to be aware of your employees having access to PHI.
Authentication methods for developing HIPAA-compliant software are as follows:
- Personal Identification Number (PIN)
- Password
- Biometrics
- Physical methods for distinguishing proof
3. Ensure transmission security
It ensures the transmission of PHI over the app network is always encrypted.
With a unique algorithm, it encrypts the PHI into a series of characters that will require a decryption key when accessing it.
You should try using it for all your communication that contains PHI.
4. Use proper disposal method for PHI
One of the HIPAA software requirements is PHI disposal.
Ensure no PHI copies are in any backup or they cannot be disposed of.
You should have preventive measures to avoid prohibited PHI disclosures and uses.
5. Ensure storage and data backup
To avoid data loss, you’ll be required to have a timely backup.
Usually, the backup is located on another data center server making it the only way to ensure maximum data security on the app.
6. Evaluate audit controls
Audits are a great and essential way for HIPAA compliance software development.
If audit controls are absent then, your application may get higher fines.
You should be aware of all the sensitive information-related operations with your mobile or web app.
7. Use encryption
To protect patient data, encryption is one of the key methods.
It not only guarantees data integrity but also allows data transmission without risk.
Cryptography, the science behind the security of messages, is the base of encryption.
Today, encryption is not just limited to personal correspondence and character conversion. It is widely used in the healthcare business.
Encryption ensures that the transmitted data is secure and safe from the eyes of hackers and intruders.
How does a healthcare compliance consultant navigate you for developing HIPAA-compliant mobile and web apps?
What does the government do? – Tell you to follow regulations.
What does a healthcare compliance consultant do? – Show you the methods to follow regulations!
Healthcare laws implementation is more important than understanding healthcare laws.
You should not only understand the HIPAA privacy laws.
But you need to develop the healthcare app in such a way that it ensures data privacy.
A healthcare compliance consultant carries out a deep-dive analysis of your app, finds security gaps, and assists the development team to fill those gaps to develop a HIPAA-compliant healthcare app.
Here, we would like to share a case study that talks about how our healthcare compliance teams helped a development firm to identify and fill 47 security gaps in the healthcare app!
You must also read: How to Hire Best HIPAA Compliance Consultant in USA, Canada?
